Table of Contents
#TGACBD to Decriminalise Cannabidiol!
YOUR HEALTH | YOUR CHOICE
On 14 November 2019, the Senate referred an inquiry into the current barriers to patient access to medicinal cannabis in Australia to the Senate Community Affairs References Committee – for inquiry and report – by 26 March 2020.
The Therapeutic Goods Administration (TGA) initiated a safety review of Cannabidiol (CBD) at lower doses, noting that there were only limited published studies available. This review commenced in late 2019 and concluded in April 2020.
The review assessed the clinical literature to investigate the safety of low doses of CBD. It investigated which low dose ranges are used in research studies. It also sought to determine if the safety profile and characteristics of the low dose CBD lends itself to consideration for down scheduling.
The review focused on safety – therefore, it did not look at the efficacy of low dose CBD for the management of specific conditions. The review also does not make specific recommendations about possible indications for low dose products.
In January 2020, the Department of Health made a submission to the inquiry. This submission acknowledged TGA’s safety review of CBD, noting that there were only limited published studies. In the submission, the Department noted that depending on the outcome of the review, the scheduling status of low dose CBD products would be considered.
The Inquiry recommended that the Therapeutic Goods Administration (TGA) conduct a public consultation on the future scheduling of cannabidiol and refer this to the Advisory Committee on Medicines Scheduling (Recommendations 12 & 13).
Next Steps
Want access to CBD? With your help, we can shape the future and bring relief to countless Australians. Submissions close Friday 22nd May.https://www.yourhealthyourchoice.com.au/tga-cbd-consultation/
Posted by Your Health Your Choice on Monday, May 18, 2020
The safety review has been completed – and the Delegate of the Secretary has now proposed to down-schedule low dose CBD.
Consultation on this proposal is now open for public comment ahead of consideration by the joint meeting of the Advisory Committee on Medicines Scheduling and the Advisory Committee on Chemicals Scheduling. The consultation closes on 22 May 2020.
FILL OUT THIS FORM – LET THE TGA KNOW HOW YOU FEEL!
Following Committee consideration at their June 2020 meeting, the Delegate will make an interim decision. A second public consultation period will then follow before the Delegate makes the final decision. More information is available here.
Shifting International Laws and Treaties: Findings of 2019 WHO ECDD Report
These shifts on the level of federal bureaucracy are not just isolated to Australia. Member states of the United Nations Commission on Narcotic Drugs (CND) has received the World Health Organisation Expert Committee on Drug Dependence’s (ECDD) cannabis recommendations.

The report recommends several changes to how cannabis is scheduled; which could have significant implications for the cannabis industry.
The scheduling of cannabis in the International Drug Control Conventions would not be nearly as restrictive as it is now. It would be removed from Schedule 4 of the 1961 Convention – the category reserved for the most dangerous substances.
THC in all forms would be removed from the 1971 Convention and placed with cannabis in Schedule 1 of the 1961 Convention – significantly simplifying cannabis classification. Pure CBD and CBD preparations containing no more than 0.2% THC would not be included in any way in the international drug control conventions. Pharmaceutical preparations containing 9-THC, if they follow certain criteria, would be added to Schedule 3 of the 1961 Convention; recognising the unlikelihood of abuse.
The report recommends cannabis and cannabis resin “be deleted from Schedule 4 of the Single Convention on Narcotics Drugs (1961).”
Schedule 4 of the 1961 Convention – the most restrictive category – includes dangerous substances with extremely limited (or no) medical value. If this recommendation is followed, cannabis and cannabis resin instead would remain in Schedule 1.
In justifying the change, the ECDD noted:
“The evidence presented to the Committee did not indicate that cannabis plant and cannabis resin were particularly liable to produce ill-effects similar to the effects of the other substances in Schedule 4 of the 1961 Single Convention on Narcotic Drugs. In addition, preparations of cannabis have shown therapeutic potential for treatment of pain and other medical conditions, such as epilepsy and spasticity associated with multiple sclerosis. In line with the above, cannabis and cannabis resin should be scheduled at a level of control that will prevent harm caused by cannabis use; and at the same time, will not act as a barrier to access, and to research and development of cannabis-related preparations for medical use.”
Further – the report recommends that Dronabinol and Tetrahydrocannabinol (THC and its isomers) both be “deleted from the Convention on Psychotropic Substances (1971) and added to Schedule 1 of the Single Convention on Narcotics Drugs (1961).” It recommends that all extracts and tinctures of cannabis be deleted from Schedule 1.
These recommendations would simplify the scheduling scheme; grouping all forms of THC in the same category as cannabis and cannabis resin. The report noted that the dangers associated with THC are similar to those of cannabis and cannabis resin; so it would be consistent to have them scheduled together.
The report compares the reclassification to cocaine being in the same category as the coca leaf, and morphine in the same category as opium.
“Due to the chemical similarity of each of the six isomers to delta-9-THC, it is very difficult to differentiate any of these six isomers from delta-9-THC using standard methods of chemical analysis,” the report said.
Doubts remain about CBD preparations containing some THC, which the report clarified its position on:
“The Committee recommended that a footnote be added to Schedule 1 of the 1961 Single Convention on Narcotic Drugs to read: ‘Preparations containing predominantly cannabidiol and not more than 0.2% of delta-9-tetrahydrocannabinol are not under international control.’”
The committee also noted:
“Cannabidiol is found in cannabis and cannabis resin; but does not have psychoactive properties […] has no potential for abuse, and no potential to produce dependence. It does not have significant ill-effects. Cannabidiol has been shown to be effective in the management of certain treatment-resistant, childhood-onset epilepsy disorders. It was approved for this use in the United States in 2018 and is currently under consideration for approval by the EU.”
TGA’s Risk-Benefit Analysis

Scheduling decisions made by bureaucracies involve a risk-benefit consideration,in the context of protecting public health.
The TGA’s risk-benefit consideration takes into account factors such as those set out in section 52E of the Therapeutic Goods Act 1989 (CTH), including the toxicity of the substance; the purpose of use (including the diagnostic decision); the potential for abuse and misuse; safety in use, including the need for specialist training or PPE; and the benefits of access to the substance.
Scheduling is a regulatory intervention to reduce public health risk to an acceptable level. Therefore, the majority of the matters detailed in section 52E(1) of the Therapeutic Goods Act 1989 (CTH), as well as the assessment factors for each schedule, relate to risk rather than benefit. However, section 52E(1)(a) requires both risk and benefit, from the use of a substance to be considered.
Where a substance is proposed to be down-scheduled, performing a risk-benefit analysis helps determine whether the substance is a suitable candidate for supply at a particular scheduling level.
The Scheduling Factors
All scheduling decisions include consideration of a standard set of factors, to ensure that public health objectives are consistently met, and the application of public health risk considerations is consistent within each Schedule. The factors for each schedule are set out in the Scheduling Policy Framework and relate back to the matters required to be taken into account by section 52E(1) of the Therapeutic Goods Act 1989 (CTH).
Amending Parts 1, 2 or 3 of the Poisons Standard
In making a decision to amend the introduction or Parts 1, 2 or 3, the Secretary considers:
- The scope of the proposed provision (e.g. whether the provision applies to poisons that are not human medicines, or to all poisons);
- The effect of the proposal on existing entries for poisons in the schedules and appendices;
- The regulatory need and justification for the change;
- The potential implications of the change for jurisdictions (e.g. compliance activities).
Amending the Schedules (Part 4 of the Poisons Standard)
When considering a new substance application, the Secretary will consider whether to include the new substance in the schedules of the Poisons Standard, based on the Scheduling factors set out in the Scheduling Policy.
If the substance is to be included in any Schedule, the Secretary will decide:
- The name or description of the substance to be used;
- The scope of the entry and the schedule(s) in which the substance is to be included;
- Which other parts of the Poisons Standard may also apply to the substance.
Rescheduling applications are only made in relation to substances that have an existing (individual substance or class) entry or entries in the Poisons Standard. When considering a rescheduling application, the Secretary will decide:
- The scope of the entry, and the schedule(s) in which the substance is to be included;
- Which other parts of the Poisons Standard may also apply to the substance.
The ‘Cascading Principle’
The model for making scheduling decisions embodies a ‘cascading principle’.

This model allows the ‘best fit’ to be found, using a systematic approach which facilitates the reclassification process; particularly when new knowledge or practices emerge which materially alter the risks to public health. This also applies when an application for rescheduling is received.
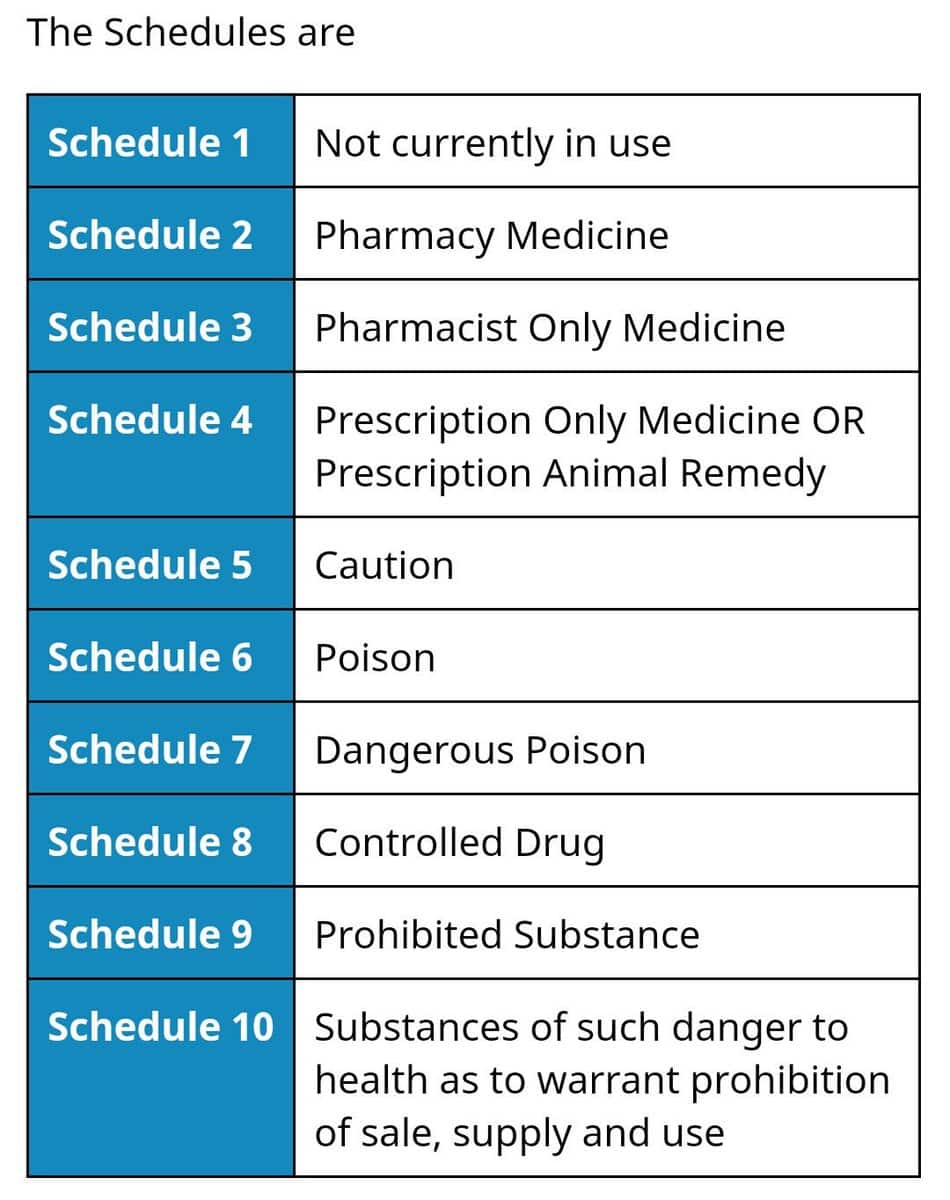
Medicines

For medicines, a substance is first assessed against the factors for Schedule 10, 9 and 8. If those factors are not applicable, the substance is assessed against the Schedule 4 factors; and if not applicable, against the Schedule 3 factors. If the Schedule 3 factors are not applicable, then the medicine is assessed against the Schedule 2 factors.
Veterinary Chemicals
Veterinary chemicals are assessed against the factors for Schedules 8 and 4 may be followed by assessment against Schedules 7, 6 and 5, as applicable. Veterinary chemicals will not be assessed against the criteria for Schedules 3 or 2.
Other Chemicals
Other chemicals are first assessed using the factors for Schedules 10 and 9; however, the highly restricted criteria for Schedule 9, relating to the propensity for dependence and abuse, means that very few substances are likely to be considered for, or included in, this schedule.
If the factors for Schedules 10 or 9 are not applicable, the substance is assessed against the Schedule 7 factors, and if not applicable, against the Schedule 6 and then the Schedule 5 factors.
Substances that are both Medicines and Chemicals
The ‘cascading principle‘ also applies to substances that are both medicines and chemicals. A substance classified into Schedule 4 when used therapeutically, may appear in Schedules 5 and/or 6 and/or 7 when intended for non-therapeutic use, or for use in the treatment of animals.
Decisions ‘Not to Schedule’
Where a substance does not meet the factors for any schedule; it should, in the interests of transparency and consistency, be listed in Appendix B. Listing in Appendix B means a decision has been made to not schedule the substance.
However, inclusion of a substance in Appendix B should not be used to infer that the substance is generally recognised as safe; in this context, it has been found to not require scheduling on the balance of risk and benefit.
Exempted From Scheduling
The term ‘exempted from scheduling’ is applicable where other forms of the same substance are listed in the schedule(s).
There are a number of mechanisms by which a substance, which appears in one or more schedules, is exempted from scheduling under certain defined circumstances. These mechanisms are:
- A general exemption for substances in Schedules 1 to 6, at a concentration not exceeding 10 mg/kg;
- An exemption for a class of products through Appendix A;
- An exemption for a substance specific to the reason for its entry in Appendix B;
- An exemption for dilute preparations through Appendix G;
- An exemption for impurities in a pesticide at a concentration at or below the maximum content for that substance specified for that pesticide in the Standards for Active Constituents, as published by the APVMA;
- A conditional exemption from within a Schedule.

An exemption may be subject to conditions including (but not limited to) maximum concentration, use, strength, dose, labelling, packaging and pack size restrictions.
With ‘Reasonable Safety’
The term ‘with reasonable safety’ means:
- The consumer is able to identify and self-manage the condition for which the medicine is intended without professional input;
- The risk of the consumer confusing their condition with more serious diseases or conditions is very small;
- The risks to health from the medicine are small and can be managed with packaging and labelling. Risks to be assessed include, but are not limited to, risks from adverse reactions, drug/food interactions and contraindications;
- The risk of inappropriate use and misuse is negligible;
- There is little need to take any special precautions in handling;
- There is net public health benefit from wider availability for the consumer.
Use the Hashtag #TGACBD – Have Your Voice Heard!